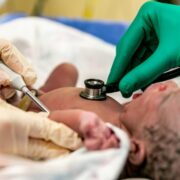
The U.S. is in dire need of more blood donations. The number of donations has dropped precipitously since the COVID-19 pandemic and the supply has been slow to rebound. But the Food and Drug Administration (FDA) has hinted that it may ease restrictions from gay and bisexual men to increase the pool of potential donors.
The FDA has long prohibited gay and bisexual men from donating blood. The agency completely prohibited men who have sex with men from donating in the 1980s due to fears that it would increase the spread of the HIV virus. In 2015, the agency revised its policy to allow gay and bisexual men to donate if they abstained from sexual contact with other men for at least 12 months. That rule was then reduced to three months amid the ongoing blood donation shortage.
But now the agency may do away with the abstention rule altogether. While the FDA has not discussed the new strategy publicly, the change in policy was first reported by the Wall Street Journal.
Under the new policy, men who have sex with men would be asked to fill out a questionnaire about their recent sexual activity and condom use before giving blood. This would allow gay men with no new partners in the last three months to donate.
Members of the LGBTQ+ community have long criticized the FDA for imposing policies that discriminate against men who have sex with men. But advocates welcomed the recent news even though the plan is still under discussion.
“I think it is a nominal step in the right direction,” said Sarah Kate Ellis, the president and chief executive of GLAAD, an LGBTQ+ advocacy organization that has been pushing to end the ban for years.
“It’s not where it should be, though. Our community and leading medical experts have been saying now for years that these decisions that the FDA is making on blood bans for the LGBTQ+ community are based in stigma and not science. And we’re seeing that pattern continue here.”
The FDA is getting ready to wrap up a study designed to assess the safety of the new blood donor screening policy. Researchers enrolled around 1,600 gay and bisexual men in eight cities across the country in hopes of coming up with a set of screening questions that would differentiate between men who are more likely to have acquired the HIV virus through sex from those who are much less likely to have the virus.
The participants answered a series of questions about their sexual history, including whether they engaged in risk-related behaviors or were taking pre-exposure prophylactic drugs, or PrEP, that reduce the risk of HIV transmission.
Brian Custer, director of Vitalant Research Institute and principal investigator of the study, said he is hopeful that the agency will have the final results by the end of the year.
“I really am confident that we have important information for the FDA to be able to consider what an individual, risk-based approach to donor selection might look like,” he said.
Susan Stramer, vice president for scientific affairs for the American Red Cross, said the study was meant “to make blood donation a more inclusive process while maintaining the safety of the blood supply.”
“While we have not been notified by the F.D.A. concerning policy changes at this time, the Red Cross looks forward to a future in which donation eligibility is not based on sexual orientation, and more healthy individuals can give blood to help patients in need,” she said.